Which Statement Best Describes a Weak Base
OI t accepts protons and completely dissociates in water. Which best statement best describes what makee a base weak.
A base is weak when its concentration is high.

. -Which statement best describes the relationship between reading. 1 point o It accepts protons and completely dissociates in water. The initial pH before the addition of any strong base is higher or less acidic than the titration of a strong acid.
It donates protons and completely dissociates in water. What is the value of the pK a that can be obtained from this titration curve. Correct answer to the question 31 32 33 34 Which statement best describes what makes a base weak.
An example of a weak base is ammonia. It accepts protons and only partially dissociates in water. There are several characteristics that are seen in all titration curves of a weak acid with a strong base.
It accepts protons and completely dissociates in water. It accepts protons and completely dissociates in water. Which Statement Best Describes a Weak Base By Ra_Kira659 18 Apr 2022 Post a Comment 14 3 Relative Strengths Of Acids And Bases Chemistry Pin On Verry True Pin On House S Adjectives Solved Which Statement Best Describes A Weak Base 1 Point Chegg Com Share.
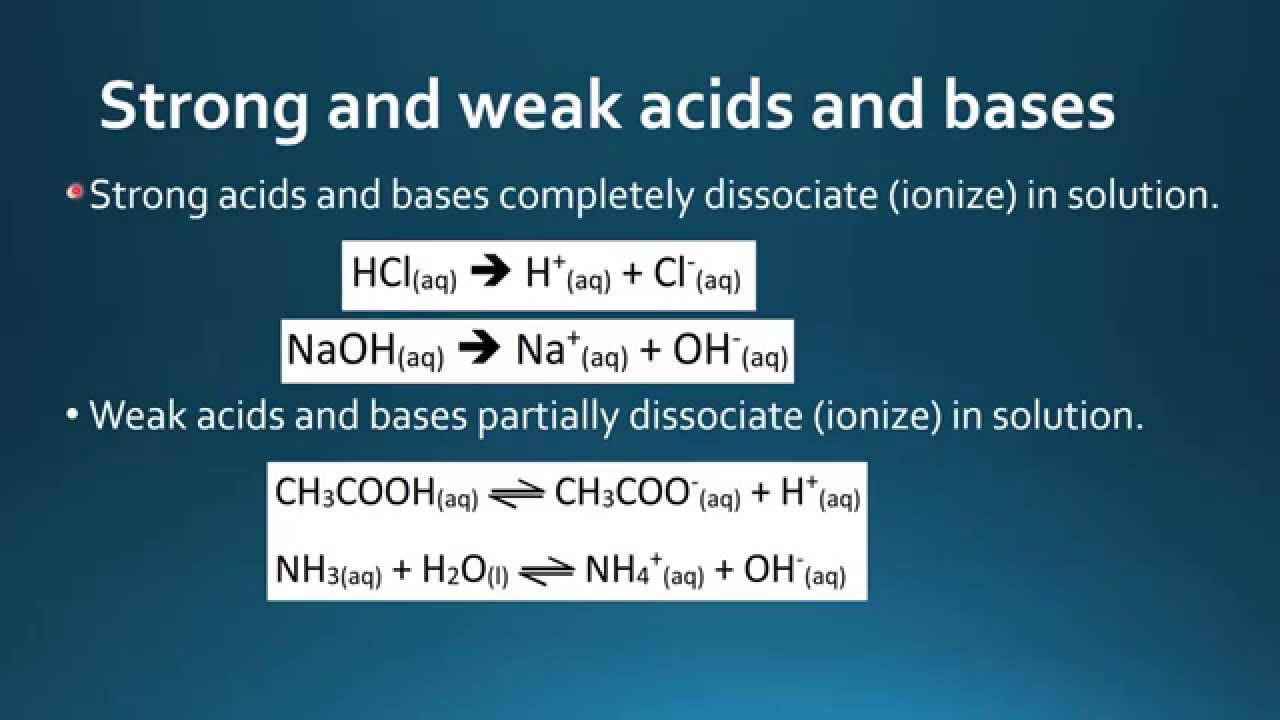
A non-protein organic molecule that is required by some enzymes in order to catalyse a reaction on a substrate describes a co-enzyme. Weak bases are the basic substances that do not completely ionize in water. There is a sharp increase in pH at the beginning of the titration.
A base is weak when it forms few ions in water. Which statement always accurately describes acids and bases a the conjugate base of a weak acid is a strong base b the conjugate acid of a. 9 Which statement best describes a weak base1 point It donates protons and only partially dissociates in water.
A non-protein substance that is required by an enzyme if it is to catalyse a reaction describes a cofactor. Which of the following statement best describe the equivalence point in a weak acid-strong base titration. The equivalence point is equal to 7.
A base is weak when it forms few ions in water. A base is weak when only a little of it is dissolved in water. A base is weak when it totally forms ions in water.
It donates protons and only partially dissociates in water. This is because sodium hydroxide undergoes almost complete ionization when it is dissolved in water. - The base word ends in a single consonant preceded by a single vowel and the suffix begins with a vowel.
A base is weak when only a little of its dissolved in water. A base is weak when its concentration is high. Choose the statement that correctly describes how a weak electrolyte looks in a beaker of water.
It must be very soluble and completely ionized D. Chemistry RI SƠ 5 months. The pH of a weak base can be anything greater than 70.
View the full answer. It donates protons and completely dissociates in water. Which statement best describes a weak base.
A 113 B 100 C 93 D 53 E 18 1 17. Decode nonsense words but is disorganized when retelling a story - a kindergartener who is good at letter naming but weak in phoneme segmentation - a second-grader. Which statement best describes a weak base.
What is the pH of a weak base. O It donates protons and only partially dissociates in water. Generally weak bases do have a lower pH that strong bases but this is not always true.
10 Which of the following descriptions best describes a prostehtic group. A Addition of a strong base to a weak acid B Addition of a weak base to a strong acid C Addition of a strong acid to a weak base D Addition of a weak acid to a strong base E Addition of a strong acid to a strong base 1 16. It accepts protons and only partially dissociates in water.
9 Which statement best describes a weak base1 point It donates protons and only partially dissociates in water. Sodium hydroxide a chemical compound with the formula NaOH is known to be a strong base. It must be of low solubility and completely ionized Homework Equations The Attempt at a Solution Homework Statement.
It may be very soluble but only partly ionized. Indicate each as either strong acid strong base weak acid or weak base. Its 010 M solution will have a pH 100 B.
A base is weak when it totally forms ions in water. Which statement best describes what makes a base weak. The equivalence point is higher than 7 due to the presence of conjugate acid from the weak base.
Ot donates protons and completely dissociates in water. It donates protons and completely dissociates in water. This is because the anion of the weak acid becomes a.
1 A weak electrolyte is mostly 95 insoluble in water so most of the solid weak electrolyte will. 1HNO3aq -- Haq NO3-aq Strong acid 2pH8 weak base. Homework Statement Which of the following best describes a weak acid A.

Ammonia Nh3 Is A Weak Base With A Kb Value Of 1 8 10 5 Dissociation Ammonia Base
8 3 3 Distinguish Between Strong And Weak Acids And Bases Youtube

Welcome To Learnapchemistry Com Ap Chemistry Question Paper Ap Chem

Strong And Weak Acids Bases Chemistry Classroom Ap Chemistry Study Tips College
Komentar
Posting Komentar